PRODUCT IDENTIFICATION
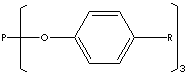
CLASSIFICATION
EXTRA NOTES
Other RN:1331-68-6, 88985-15-3, 125935-91-3, 221006-80-0
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
USA.gov - Tris(nonylphenyl )phosphite
Wikipedia Linking - Tris(nonylphenyl )phosphite
Google Scholar Search - Tris(nonylphenyl )phosphite
PubChem Compound Summary - Tris(nonylphenyl )phosphite
KEGG (Kyoto Encyclopedia of Genes and Genomes) - Tris(nonylphenyl )phosphite
ChEBI (http://www.ebi.ac.uk/chebi/) - Tris(nonylphenyl )phosphite
NCBI (http://www.ncbi.nlm.nih.gov/) - Tris(nonylphenyl )phosphite
EPA - Substance Registry Services - Tris(nonylphenyl )phosphite
U.S. Environmental Protection Agency
Hazard Characterization Document Tris(nonylphenyl) Phosphite (CASRN 26523-78-4)
Local:
Phosphorous acid is a clear to yellowish crystalline solid with a garlic like odour melting at 73 C, decomposes at 200 C. It is very soluble in water and in alcohol. This compound contains one direct P-H bond (which is not very acidic) and only two hydrogens bonded to oxygen (which are acidic). The structure of this material is more correctly written (HO)2HPO. For this reason, this dibasic acid forms two series of salts, one containing the dihydrogen phosphite ion, H2PO3- , and the other containing the hydrogen phosphite ion, HPO32-. It is prepared by hydrolysis of phosphorus trichloride (or tetraphosphorus hexaoxide) with alcohols or phenols. Phosphorous acid esters are called phosphite with the formula (RO)3P. Phosphorous acid and phosphite are used as reducing agents in chemical industry because of easy oxidation property to phosphoric acid. They are used as antioxidant, stabilizer and chelating agent in plastic system. They are used as solvent in paint and as flame retardant on fibres. They are used as a chemical intermediate in the production of pharmaceutical ingredients, pesticides, optical brighteners and in lubricant additives and adhesives. Tris(nonylphenyl) Phosphite is used as a heat stabilizer for polymers especially for unvulcanized rubbers and polyvinylchloride. It is a phenolic antioxidant maintain the performance integrity, color stability and processing stability of ABS, polycarbonate, polyolefins and vulcanized rubber.
Heat stabilizer for polymers. Many polymers are susceptible to environmental degradation and require the addition of a stabilizer such as an antioxidant or UV absorber to prevent premature degradation. Hindered phenols and hindered amine light stabilizers (HALS) deactivate existing radicals, including peroxy compounds formed by air oxidation. UV absorbers dissipate UV radiation by a process nondestructive to the polymer. Free radical inhibitors are added to monomers as stabilizers to inhibit premature polymerization.
APPEARANCE
REFRACTIVE INDEX
ACID NUMBER
COLOR
1 max (Gardner)
HEAT LOSS
0.5% max
HAZARD OVERVIEW
GHS (Globally Harmonised System) Classification: Not a dangerous substance. Potential Health Effects: Eyes - May cause eye irritation. Skin - May be harmful if absorbed through skin May cause skin irritation. Inhalation - May be harmful if inhaled. May cause respiratory tract irritation. Ingestion - May be harmful if swallowed.
RISK PHRASES
36/37/38
SAFETY PHRASES
26-36